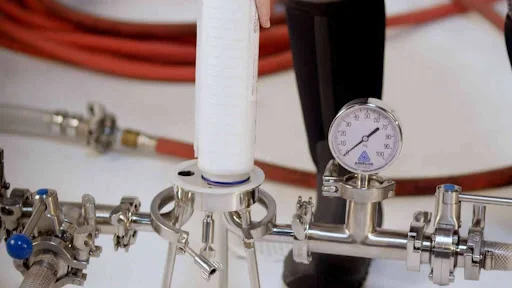
Filtration systems are critical in industries ranging from pharmaceuticals and biotechnology to water treatment and chemical processing. Ensuring that filters function as intended is not just about maintaining efficiency—it is also about safety, regulatory compliance, and protecting the quality of the final product. One of the most vital steps in ensuring filter performance is filter integrity testing. This guide provides an in-depth exploration of filter integrity testing, its types, methodologies, applications, and practical tips for achieving optimal results.
1. What is Filter Integrity Testing?
Filter integrity testing is the process of verifying that a filter, particularly a membrane filter, is functioning correctly and has no defects that could compromise its performance. Unlike routine maintenance or visual inspection, integrity testing provides quantitative evidence that the filter meets required specifications.
Integrity testing is critical in systems where sterility or high purity is essential. For example, in biopharmaceutical production, a compromised filter could allow microbial contamination, causing product recalls or even posing safety risks to patients.
There are two primary types of filters commonly subjected to integrity testing:
- Hydrophilic filters – used for aqueous solutions and characterized by their high wettability.
- Hydrophobic filters – often used in gas filtration or for liquids that are not easily wetted.
Regardless of the type, integrity testing helps identify:
- Pinhole defects
- Incomplete membrane sealing
- Damage due to mishandling or pressure variations
2. Importance of Filter Integrity Testing
Filter integrity testing is more than just a quality check. It ensures:
- Product Safety and Quality: A filter that is compromised can allow contaminants to pass through, which is unacceptable in pharmaceuticals, food, and beverage industries.
- Regulatory Compliance: Agencies such as the FDA, EMA, and WHO mandate integrity testing for sterilizing-grade filters before and after use in critical processes.
- Operational Reliability: Detecting defects early prevents costly downtime and contamination events.
- Cost Efficiency: Early detection of compromised filters reduces waste and avoids potential product recalls.
A widely recognized method in the industry is the bubble point test, which provides reliable confirmation that a filter’s pores are intact and that it maintains the required retention capability. More about this method can be found in the article The Importance of Bubble Point Test for Filter Integrity.
3. Types of Filter Integrity Tests
Filter integrity testing generally falls into two broad categories:
3.1 Non-Destructive Tests
Non-destructive tests verify filter integrity without affecting its usability. This is especially crucial for expensive or single-use filters. Key non-destructive methods include:
- Bubble Point Test: Measures the pressure at which a liquid is forced out of the largest pores in the membrane, indicating pore integrity.
- Widely used for sterilizing-grade membrane filters.
- Provides precise data on pore size uniformity.
- Reference: Procedure for Bubble Point Filter Integrity Test.
- Widely used for sterilizing-grade membrane filters.
- Diffusion Test (Forward Flow or Pressure Hold Test): Detects leaks by monitoring the flow of air or liquid through the wetted filter under controlled pressure conditions.
3.2 Destructive Tests
Destructive tests sacrifice the filter to evaluate its maximum performance. They are primarily used for validation and development purposes. Common destructive methods include:
- Microbial Challenge Test: Involves passing a known concentration of microorganisms through the filter to verify retention capability.
- Bubble Point Confirmation on Cut Sample: Physically measures pore sizes after sectioning the filter.
4. Key Considerations Before Testing
Before conducting filter integrity tests, it is essential to consider:
- Filter Type and Material: Polyethersulfone (PES), polyvinylidene fluoride (PVDF), and nylon membranes have different wettability and chemical compatibility, affecting test methodology.
- Fluid Type: Water, alcohol, or other test liquids may affect results due to differences in surface tension.
- Test Equipment Calibration: Accuracy of pressure gauges and flow meters is critical for reliable results.
- Environmental Conditions: Temperature, pressure, and humidity can influence test outcomes.
5. The Bubble Point Test in Detail
The bubble point test is a cornerstone of non-destructive filter integrity testing. It is based on the principle that the largest pore in a wetted membrane will allow gas to pass through at a specific pressure. The steps are as follows:
- Wetting the Filter: The membrane is fully saturated with a compatible liquid.
- Applying Gas Pressure: Gas (usually air or nitrogen) is gradually applied to one side of the filter.
- Detecting Bubbles: The pressure at which the first continuous stream of bubbles appears on the downstream side is recorded.
- Comparing to Specifications: The observed bubble point is compared to the manufacturer’s minimum bubble point specification.
Why the Bubble Point Test is Reliable:
- Detects the largest pore or pinhole defect.
- Non-destructive and reproducible.
- Required by regulatory authorities for sterilizing-grade filters.
For a detailed procedural guide, refer to Procedure for Bubble Point Filter Integrity Test.
6. Diffusion or Pressure Hold Test
Another common non-destructive test is the pressure hold test, also called the diffusion test. In this method
- The filter is wetted completely.
- A constant pressure is applied to one side of the filter.
- Flow is measured over a specific time period.
If the flow exceeds the acceptable limit, the filter is considered compromised. This test is often used when the bubble point test is unsuitable, such as with certain single-use or low-porosity membranes.
7. Filter Integrity in Different Industries
7.1 Biopharmaceuticals
- Ensures that sterilizing-grade filters effectively remove bacteria and endotoxins.
- Mandatory pre-use and post-use integrity testing.
- High risk if testing is skipped: contamination can result in massive recalls or patient risk.
7.2 Food and Beverage
- Ensures microbial control in beverages, dairy, and water filtration.
- Non-destructive integrity tests allow filters to be reused without compromising safety
7.3 Water Treatment and Chemical Processing
- Removes particulates, bacteria, and other contaminants.
- Helps in meeting regulatory and environmental standards.
- Integrity testing ensures long-term reliability in high-volume systems.
8. Common Challenges in Filter Integrity Testing
While filter integrity testing is crucial, it is not without challenges:
- Incorrect Wetting: Improper wetting can lead to false failures.
- Operator Error: Inconsistent pressure application or timing errors may skew results.
- Temperature Effects: Temperature variations can change surface tension, affecting bubble point readings.
- Instrument Calibration: Uncalibrated or poorly maintained instruments compromise reliability.
Mitigating these challenges requires proper training, adherence to manufacturer guidelines, and routine equipment calibration.
9. Best Practices for Reliable Testing
- Always follow the manufacturer’s recommended test procedure.
- Use compatible liquids for wetting the filter.
- Ensure the test equipment is calibrated and functioning correctly.
- Record all pressure, flow, and time data meticulously.
- Implement pre-use and post-use testing to monitor filter performance over its lifecycle.
- Maintain documentation for regulatory compliance and quality assurance audits.
10. Advanced Techniques and Automation in Filter Integrity Testing
As industries demand higher efficiency, reliability, and compliance, advanced technologies have emerged to enhance filter integrity testing. Automation has transformed the process, offering reproducible and faster results while minimizing human error.
10.1 Automated Bubble Point Testing
Automated bubble point testers use sensors and digital pressure controllers to monitor bubble formation accurately. Advantages include:
- Higher Precision: Digital sensors detect minute bubbles, reducing false positives.
- Data Logging: Automatic recording of pressure, flow, and temperature enables traceability.
- Reduced Operator Dependency: Minimizes errors due to inconsistent pressure application or timing.
This method is especially critical in pharmaceutical production, where consistency and regulatory compliance are paramount.
10.2 Remote Monitoring and Online Integrity Testing
Modern filtration systems in water treatment or large-scale production facilities can integrate online integrity testing. This technique allows continuous monitoring without disassembling the filter:
- Measures differential pressure and flow continuously.
- Alerts operators when filters approach integrity limits.
- Enhances preventive maintenance and avoids unexpected downtime.
10.3 Single-Use Systems Integrity Testing
Single-use filtration systems have gained popularity in biopharmaceutical production due to reduced cleaning requirements and cross-contamination risk. For these systems:
- Pre-sterilized, disposable filters are integrity tested before use.
- Non-destructive tests like bubble point and diffusion tests are preferred, ensuring the filter remains usable.
- Integration with automated systems facilitates rapid testing during production.
11. Case Studies of Filter Integrity Testing
11.1 Biopharmaceutical Case Study
A biopharmaceutical company manufacturing vaccines relied on 0.2 µm sterilizing-grade filters. By implementing bubble point testing both pre-use and post-use, the company:
- Detected a compromised filter before production begn, avoiding contamination.
- Improved batch consistency and reduced product loss.
- Ensured compliance with FDA regulations for sterile filtration.
Reference: The Importance of Bubble Point Test for Filter Integrity
11.2 Water Treatment Plant Example
In a municipal water treatment plant, integrity testing of ultrafiltration membranes:
- Prevented potential microbial contamination.
- Enabled predictive maintenance, reducing unplanned shutdowns.
- Enhanced public safety by maintaining consistent water quality.
12. Regulatory Compliance and Documentation
Regulatory authorities worldwide emphasize the importance of filter integrity testing in critical applications. Key guidelines include:
- FDA Guidance for Sterile Drug Products: Requires pre- and post-use integrity testing of sterilizing-grade filters.
- USP <1231>: Details filtration integrity test procedures, including bubble point and diffusion tests.
- EMA Guidelines: Recommends validation of sterilizing filters and documentation of test results.
Proper documentation should include:
- Filter manufacturer and model
- Test method and procedur
- Date and operator information
- Test results (bubble point, pressure hold, or diffusion)
- Any corrective actions taken
This documentation not only ensures compliance but also supports quality audits and traceability in case of product investigation.
13. Troubleshooting Common Issues in Integrity Testing
Even with precise methods, testing may occasionally yield unexpected results. Common troubleshooting steps include:
- Low Bubble Point Reading:
- Ensure proper wetting of the filter.
- Check for leaks in the test setup.
- Verify test liquid compatibility with the membrane.
- Ensure proper wetting of the filter.
- High Diffusion Flow Rate:
- Confirm that the filter is properly seated in the holder.
- Inspect for mechanical damage or pinholes.
- Ensure consistent test pressure and temperature.
- Confirm that the filter is properly seated in the holder.
- Operator Errors:
- Provide proper training on bubble point and pressure hold tests.
- Maintain standardized procedures to reduce variability.
- Provide proper training on bubble point and pressure hold tests.
14. Filter Integrity Testing Best Practices
For maximum effectiveness, filtration system managers should adopt these practices:
- Pre-Use Testing: Conduct bubble point or diffusion tests on new filters before introducing process fluids.
- Post-Use Testing: Verify filter integrity after use to confirm no damage occurred during operation.
- Calibration and Maintenance: Regularly calibrate pressure gauges, flow meters, and automated testers.
- Standard Operating Procedures (SOPs): Ensure all operators follow documented and validated test procedures.
- Integration into QA Programs: Use test data for quality assurance and regulatory audits.
- Use of External References and Training: Leverage industry knowledge such as Procedure for Bubble Point Filter Integrity Test to ensure best practices.
15. Emerging Trends in Filter Integrity Testing
The filtration industry continues to innovate, and several trends are shaping the future of integrity testing:
- Digital Twin and AI Integration: Advanced AI algorithms predict filter failure and optimize testing schedules.
- Real-Time Data Analytics: Online monitoring combined with cloud-based analytics provides predictive maintenance insights.
- Environmentally Friendly Practices: Development of biodegradable test liquids and reusable filter components reduces environmental impact.
- Miniaturized Testing Devices: Compact, portable integrity testers enable on-site testing in remote or small-scale facilities.
These trends improve safety, efficiency, and compliance while reducing operational costs.
16. Conclusion
Filter integrity testing is an essential component of any filtration system. It guarantees that filters perform as intended, protecting product quality, ensuring safety, and maintaining compliance with regulatory standards. Key points to remember:
- Non-destructive tests like bubble point and pressure hold tests are critical for pre-use and post-use validation.
- Automation and online monitoring enhance reliability and reduce human error.
- Proper documentation, calibration, and adherence to SOPs ensure accurate results and regulatory compliance.
- Emerging trends like AI integration and real-time analytics will continue to improve integrity testing practices.
By implementing a rigorous filter integrity testing program, industries can achieve higher reliability, reduce contamination risk, and maintain consistent product quality. Whether for pharmaceuticals, water treatment, or chemical processing, filter integrity testing is a non-negotiable step in modern filtration operations.
For additional reading on critical aspects of filter integrity testing, consult:
- The Importance of Bubble Point Test for Filter Integrity
- Procedure for Bubble Point Filter Integrity Test – Econe Filtration